Bringing medicine into Japan requires careful attention, as certain prescription and over-the-counter medications that are commonly used in other countries may be strictly prohibited. The Japanese government determines which medications and medical devices can be legally imported, and these regulations can change at any time. Before traveling to Japan, it’s important to stay informed by checking the latest official guidelines and how they may apply to your medication.
Prescription Medications
Stimulants, including heroin, cocaine, MDMA, opium, cannabis (marijuana), and some prescription medications, such as Adderall, are strictly prohibited in Japan, even if the substances were obtained legally outside of Japan. Japanese customs officials or police will detain travelers importing illegal items.
Up to one month’s supply of prescription medicine (permissible under Japanese law) can be brought into Japan. Travelers should prepare a copy of their doctor’s prescription as well as a letter stating the purpose of the drug.
Those who must carry more than one month’s supply, or are carrying syringes (pumps) or a CPAP machine, must obtain a “Yunyu Kakunin-sho*” (formerly referred to as the “Yakkan Shoumei”), import certificate in advance, and present it along with their prescription medicines at Customs upon arrival in Japan.
*Please be advised that “Yunyu Kakunin-sho” certificate applications should be applied for at least two weeks before your travel date to Japan.
Over-The-Counter Medicines
Japanese laws stipulate that some over-the-counter medicines, including types of inhalers, allergy and sinus medications, are illegal. Specifically, products that contain stimulants or Codeine are prohibited if they contain more than an allowed quantity of the stimulant ingredient. This includes medicines that contain Pseudoephedrine, such as Actifed, Sudafed, and Vicks inhalers.
According to Japanese law, a two-month supply of allowable over-the-counter medication and a four-month supply of permitted vitamins can be brought into Japan duty-free.
Please note that disposable contact lenses are recognized as over-the-counter medication and must be prepared and declared as such.
As the list of permitted medications in Japan is subject to change at any time, we strongly recommend checking the latest official information on the Ministry of Health, Labour and Welfare (MHLW) website here.
Accessible Code to Japanese OTC Medicines
Since 2020, a few different pharmaceutical companies in Japan have started adding Accessible Code to their over-the-counter (OTC) medicine packaging. This is a groundbreaking initiative aimed at enhancing accessibility for individuals with visual impairments, and it also benefits people who need translations for products commonly sold in Japanese drug stores. Accessible Code employs QR codes that, when scanned, provide text and audio playback of essential product information in up to 15 languages, automatically selecting the language based on the user’s device settings. This innovation allows users to access details about ingredients, dosage instructions, safety precautions, and more, ensuring that vital information is available to all consumers, regardless of their physical or language abilities.
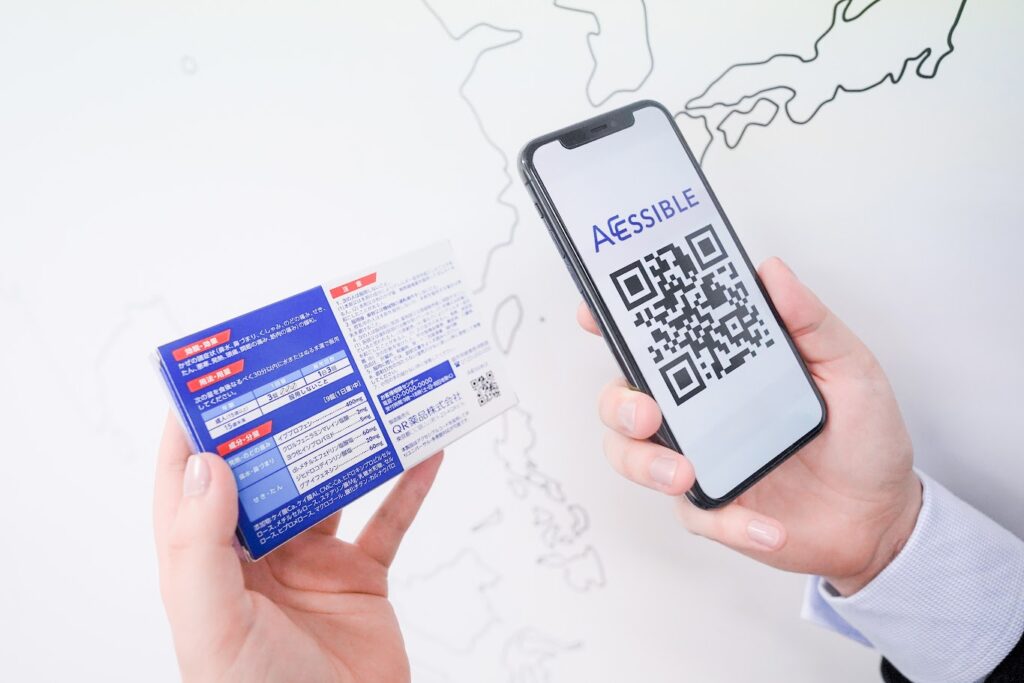
Image © Accessible Code. This is a sample to show how the code works and does not represent a real product.
